
1-HOUR NECK PROCEDURE


1-HOUR NECK PROCEDURE

PHYSICIAN FINDER
Enter your Name, Email and Zip Code below to find physicians near you that use Renuvion.
PHYSICIAN FINDER
Enter your Name, Email and Zip Code below to find physicians near you that use Renuvion.
PLASTIC SURGEON
DR. PAUL NASSIF
Shares his thoughts on Renuvion and the 1-Hour Neck Contouring procedure.
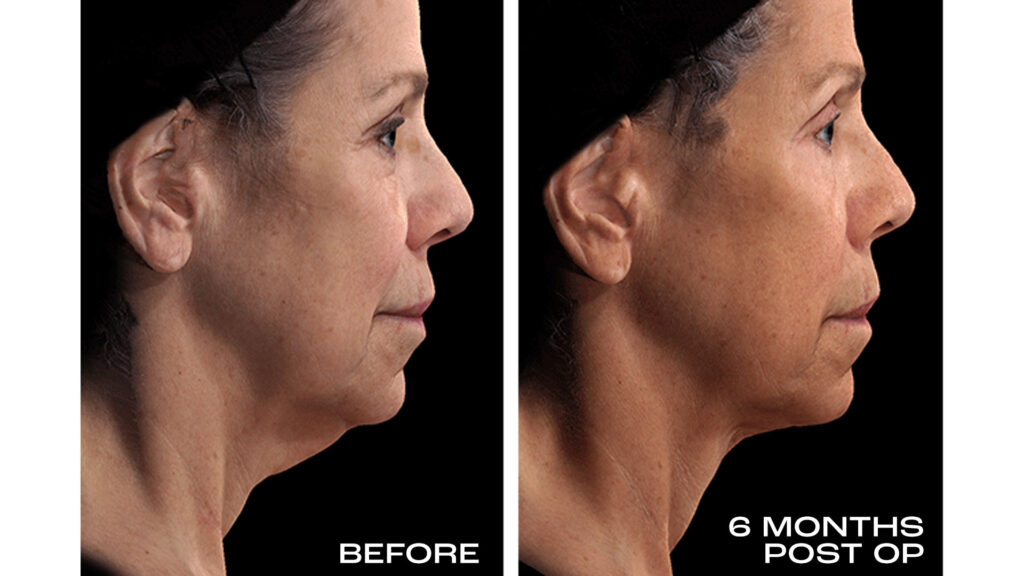
65-YEAR-OLD FEMALE
REAL RESULTS
Results from IDE Clinical Study #G190152 where Renuvion was the only technology used.
RENUVION
HOW IT WORKS
Renuvion is a minimally-invasive procedure that treats the collagen fibers under the skin, contracting them and triggering the collagen rebuilding process known as neocollagenesis.¹ This procedure smooths and contours the neck and chin for an overall younger and tighter-looking appearance.²
EXPERIENCE
THE BENEFITS
- 1-Hour Procedure
- Minimally Invasive
- Single-Treatment Solution
- Minimal Discomfort
- Short Recovery Time
- Visible Results Quickly
- Long-Term Results
REFERENCES:
1. Duncan DI and Roman S. Helium Plasma Subdermal Tissue Contraction Method of Action. Biomed J Sci & Tech Res 31(2)-2020. BJSTR. MS.ID.005075.
2. Clinical Data on File, IDE G190152/NCT04146467. G190152. https://www.clinicaltrials.gov/ct2/results?term=NCT04146467
3. Renuvion Physician Survey Results, MM0317.01 0422 – https://www.renuvion.com/wp-content/uploads/2022/05/renuvion-physician-survey-results-brochure_mm0317.01_050222.pdf.
The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma for cutting, coagulation and ablation of soft tissue during open surgical procedures. The Renuvion APR Handpiece is indicated for use in subcutaneous dermatological and aesthetic procedures to improve the appearance of lax (loose) skin in the neck and submental region.
The Renuvion APR Handpiece has general indications for use as well as indications to improve the appearance of lax skin in the neck and submental region only. The Renuvion APR Handpiece has not been cleared or approved for use with liposuction or in combination with any aesthetic procedure. As with any aesthetic procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use. Risk associated with the use of the Renuvion APR may include: helium embolism into the surgical site due to inadvertent introduction into the venous or arterial blood supply system, unintended burns (deep or superficial), pneumothorax, temporary or permanent nerve injury, ischemia, fibrosis, infection, pain, discomfort, gas buildup resulting in temporary and transient crepitus or pain, bleeding, hematoma, seroma, subcutaneous induration, pigmentation changes, increased healing time, scarring, asymmetry and/or unacceptable cosmetic result. Expected clinical side effects may include discomfort/pain, edema, erythema, ecchymosis, hypoesthesia, touch sensitivity, itching, temporary weight gain, temporary numbness/tingling, subcutaneous nodules or lumps (transient migratory firmness), temporary and/or transient crepitus. Please see the instructions for use for more detailed information.






